By Chris Faubel, MD —
An interesting blurb about the discovery of corticosteroids can be found here .
Common Corticosteroid Preparations
- Methylprednisolone acetate = Depo-Medrol
- Triamcinolone acetonide = Kenalog
- Betamethasone acetate + betamethasone sodium phosphate = Celestone Soluspan
- Dexamethasone sodium phosphate = Decadron
Steroid Properties
- Soluble versus Insoluble (in water)
- Insoluble
- The acetate/acetonide variety are insoluble, because they contain steroid ester groups, which leads to microcrystallization in water.
- Examples: Methylprednisolone, Triamcinolone, Betamethasone acetate
- Therefore, all of these vials need to shaken to mix the steroid with the solution before drawing up into the syringe.
- Soluble
- Non-esters; not particulates in water
- Dexamethasone is the only commercially available soluble glucocorticoid.
- Compounding pharmacies can make betamethasone sodium phosphate in individual vials for you.
- What’s the advantage of having the insoluble steroids with esters groups?
- These corticosteroid esters require hydrolysis by cellular esterases in order to release the active part –> last longer in the joint than nonesters
Advantages of the Non-Ester Preparations
- Taken up quickly by the cells and therefore have a more rapid onset.
- But then also don’t last as long, because they get washed away from the area quickly.
Advantages of the Ester
- Since they require our cells to first hydrolyze them with esterases to release the active part –> expected to have a longer duration of clinical efficacy
- Unfortunately, no studies have shown this clinically.
Particle Size and Aggregations
- When these steroid preparations are mixed with blood, they can clump together to form aggregates. This is particularly important when performing cervical transforaminal epidural steroid injections (C-TFESIs).
- Drs. Derby and Tiso both conducted studies to examine the particle size and amount of aggregation with the various preparations. [1,2]
- Overall, the ester formulations have much more likelihood of forming aggregations, and are therefore not advised to be used for C-TFESIs.

Derby et al. “Size and aggregation of corticosteroids used for epidural injections”. Pain Med 2004;5:202-5
- Risk of Aggregations
- The steroid aggregations are thought to explain the brain and spinal cord infarctions that has been seen after cervical TFESIs.
Relative Potencies
- The dosages for Decadron and Celestone Soluspan are 5x less than Depo-medrol and Kenalog.

Contraindicatons to Using Injectable Corticosteroids
- Local or intraarticular sepsis
- Avoid if ANY infection of joint or nearby skin
- Bacteremia
- Avoid if currently septic (duh), or may likely become septic (pneumonia, endocarditis)
- Intraarticular fracture
- Corticosteroids inhibit bone healing
- Joint instability
- Avoid in joints that are already unstable
- Steroids are thought to cause MORE instability by causing subchondral osteonecrosis and weakening of the capsule and ligaments
- Severe juxtaarticular osteoporosis
- Some think corticosteroids can decrease bone density even more, with resultant fracture
- Others feel the already present synovitis causes the osteoporosis, and that attenuating that with corticosteroids will improve the osteoporosis/osteopenia
- No published reports to support this hypothesis
- Warfarin use
- A study by Thumboo et al, showed ZERO complications in patients with a mean INR of 2.6 [4]
- Because of the small ‘n’ in that study (only 32 joints or soft tissue injections/aspirations), some recommend not performing on patients with INR greater than 2.
Adverse Effects
- Septic arthritis
- The most feared complication from joint injections
- With good sterile technique, incidence is as low as 0.01%-0.03% [5]
- Based on the current existing literature, use of clean technique prior to joint injection seems to be comparable to sterile technique in safety and can be performed at lower cost.Clean technique being defined as use of handwashing, non-sterile gloves, and alcohol swab prior to injection.
- Post-injection flare
- Most common adverse effect
- Local increase in inflammation that develops within hours and can last 2-3 days
- If severe, may be difficult to distinguish b/t sepsis –> may need aspiration of joint
- Prevalence is 2-25%
- Note: NO correlation b/t flare and response to injection
- Causes (theories)
- Crystallization from ester preparations
- Response to other chemicals (benzyl alcohol, polyethylene glycol)
Clinical pearl:
If this occurs in a patient, try using one of the non-particulate, non-ester formulations
- Articular hyaline cartilage damage
- Controversial and conflicting data
- Results of animal studies are ambiguous [6]
- All studies looking at joint cartilage were animal models
- Two rabbit studies showed reduction in cartilage production .
- One in dogs showed a protective effect.
- One in horses showed increased cartilage turnover
- A recent retrospective review of human xrays showed no deleterious effects on joint
- Despite case reports of severe arthropathy, most studies on humans suggest that, when used appropriately, the beneficial effects of intra-articular corticosteroids exceed the harmful effects
- It is recommended that corticosteroid injections into the same joint should be limited, for instance to 1 injection every 6 weeks and no more than 3 to 4 in one year
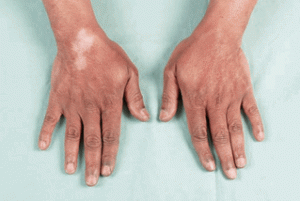
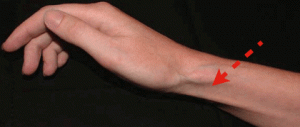
- Skin atrophy and Depigmentation
- Most noticeable after injection of superficial structures (DeQuervain’s tenosynovitis, lateral/medial epicondyle, neuroma, pes anserine bursa, etc.)
- May also be seen with joint injections; likely due to tracking of steroid along the needle path when withdrawing the needle.
- Solution: Empty the syringe completely before withdrawing. Also, replace the stylet of spinal needle before withdrawing.
- Cause is not known
- Theories include vasoconstriction and decreased type I collagen and glycosaminoglycan synthesis.
- Clinical Pearl: Stay away from Kenalog for superficial injections
- Depigmentation
- May take up to 2 months to develop

- More common with long-acting agents (kenalog and depomedrol)
- Greater incidence in dark-skinned patients
- Returns to normal in most people over a 12-month period
- May be accelerated by UV light exposure
- May take up to 2 months to develop
- Skin atrophy
- Tendon rupture
- Animal studies have shown that intratendinous injection of steroids adversely affect the biomechanical properties
- Steroid effects on isolated collagen fascicles (in vitro) has shown reduction in their strength
- Note: No in vivo studies of humans have shown that peritendinous injections predispose to ruptures
- Adrenal suppression
- Soft tissue and intraarticular injections can have a measurable effect on the hypothalamus-pituitary-adrenal axis
- Study:
- Seven of 10 athletes had abnormally low serum cortisol levels after a single intraarticular steroid injection
- Also had blunted response to exogenous ACTH (adrenocorticotropic hormone)
- Lasted 14 days in some of the athletes
- End result is a decreased ability to respond to stress
Advice:
No surgery, severe dehydration, or severe physical stress for two weeks after an injection
- Hyperglycemia
- Diabetic patients can expect an increase in blood glucose for 2-5 days after the injection
As an aside:
A couple studies have shown corticosteroid injections to be less effective in diabetic patients
- Facial flushing
- Although not dangerous, facial flushing is an unpleasant effect
- 15% of patients
- Occurs 2-30 hours after the injection
- Can last 36 hours
- More common with Kenalog (but can happen with all of them)
- May be accompanied by chilliness, shaking, headaches
- Likely a histaminic response – can pre-treat with 25-50mg of benadryl, or switch to another corticosteroid
Adverse CNS Sequelae
- Brain and spinal cord infarcts
- Mostly from cervical TFESIs, with inadvertent emboli from particulate steroids into radicular/ascending cervical/deep cervical arteries supplying blood to the spinal cord and brain
- No studies or case reports have shown adverse CNS effects from non-particulates (betamethasone sodium phosphate & dexamethasone sodium phosphate)
- Note: Dreyfuss and Bogduk published a study showing no statistically or clinically significant difference b/t particulate and non-particulate preparations for cervical radiculopathy [Pain Medicine 2006;7:237-242]
Suggestions:
- Use only non-particulates (compounded BSP or dexamethasone sodium phosphate) for cervical, thoracic, and upper lumbar TFESIs
- For superficial injections, avoid triamcinolone (Kenalog)
Other Potential Sources of Adverse Effects
- Benzyl alcohol – a preservative
- Neurotoxic effects (demyelination, neural degeneration, paraplegia)
- Not thought to be a problem b/c the CNS effects are too sudden
- Polyethylene glycol – a drug vehicle
- Some feel it has no neurotoxic effects at commercially available concentrations
————————————————————————————-
REFERENCES:
1 – Tiso et al. “Adverse central nervous system sequelae after selective transforaminal block: the role of corticosteroids”. Spine Journal. 2004;4:468-74
2 – Derby et al. “Size and aggregation of corticosteroids used for epidural injections”. Pain Med 2004;5:202-5
3 – MacMahon PJ, Eustace SJ, Kavanagh EC. “Injectable Corticosteroid and Local Anesthetic Preparations: A Review for Radiologists”. Radiology 2009;252(3):647-661
4 – Thumboo et al. “A prospective study of the safety of joint and soft tissue aspirations and injections in patients taking warfarin sodium”. Arthritis Rheumatism. 1998;41:736-739
5 – Baima and Isaac. “Clean versus sterile technique for common joint injections: a review from the physiatry perspective”. Current Reviews in Musculoskeletal Medicine. 2008 June; 1(2): 88-91
6 – Ostergaard et al. “Intra-articular corticosteroids in arthritic disease: a guide to treatment”. BioDrugs 1998;9:95-103
7 – Rogojan et al. “Depigmentation: a rare side effect to intra-articular glucocorticoid treatment”. Clinical Rheumatology 2004;23:373-375






Dr. Faubel is da man!
I received a steroid injection in my left wrist area for severe pain in September of 2013. It is now December 2014, and I have severe subcutaneous atrophy. The skin in that area is pink/red and is VERY SENSITIVE. I can’t wear long sleeve shirts or jewelry on my wrist. I visited several hand specialists who inform me that the atrophy is permanent and that this is the most severe atrophy they have seen due to a steroid injection. My life is forever changed…. Every day I experience a new sensation in my wrist and hand: burning, zings, and aches. In addition to having to look at an atrophied wrist/forearm that continuously needs babied as any grazing results in severe pain and bruising similar to an elderly person on coumarin. You state in your article that “cutaneous atrophy normalizes.” Is this true? Do I have ANY chance of gaining some SQ tissue to protect my normally petite wrist?? I am desperate to find someone who can help with my SQ atrophy as it’s making my life miserable. Thank you
I have seen some talk about injecting normal saline into the atrophied area repeatedly (every month, I believe) for a few months in a row and that helped. May be worth investigating.