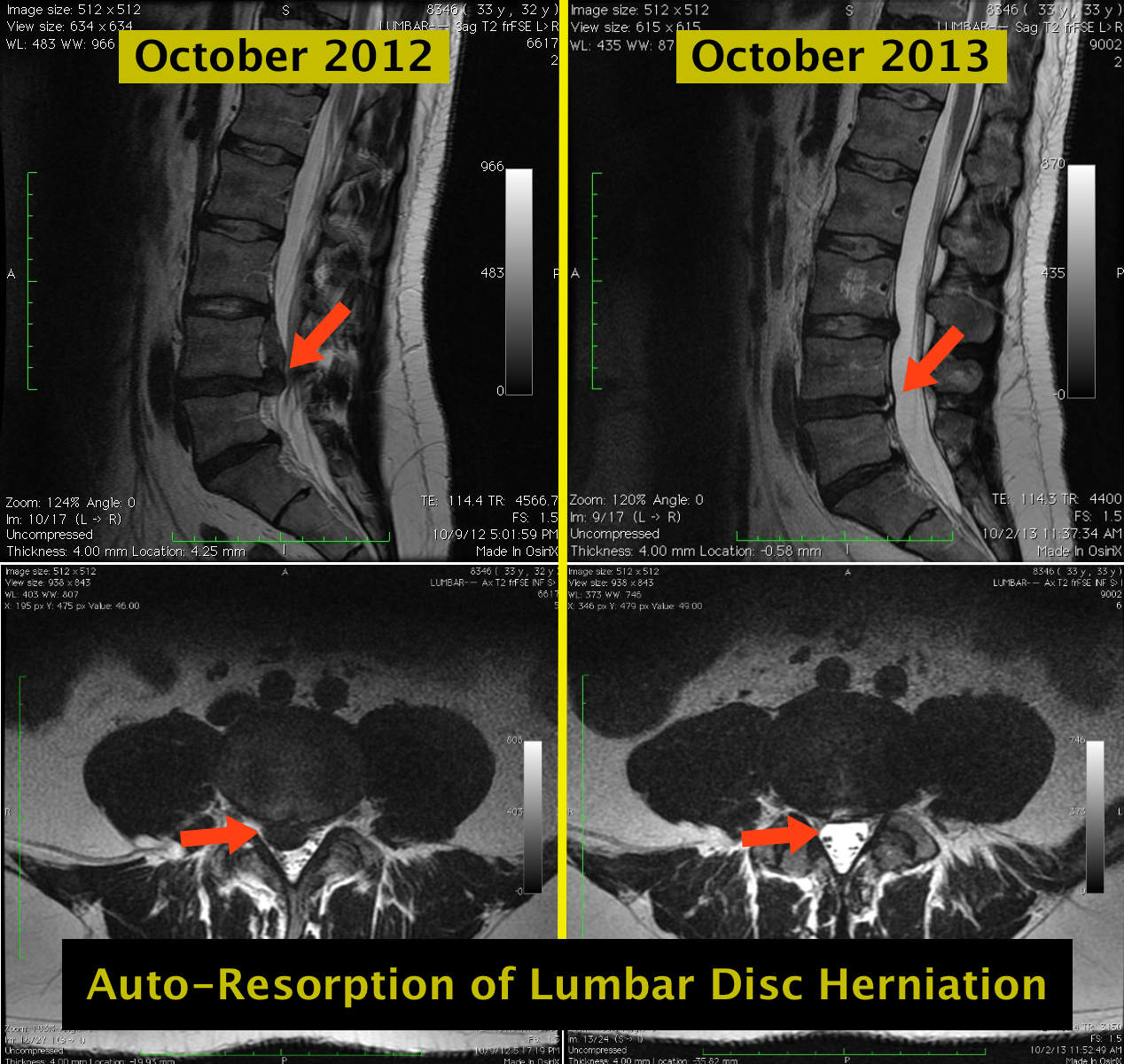
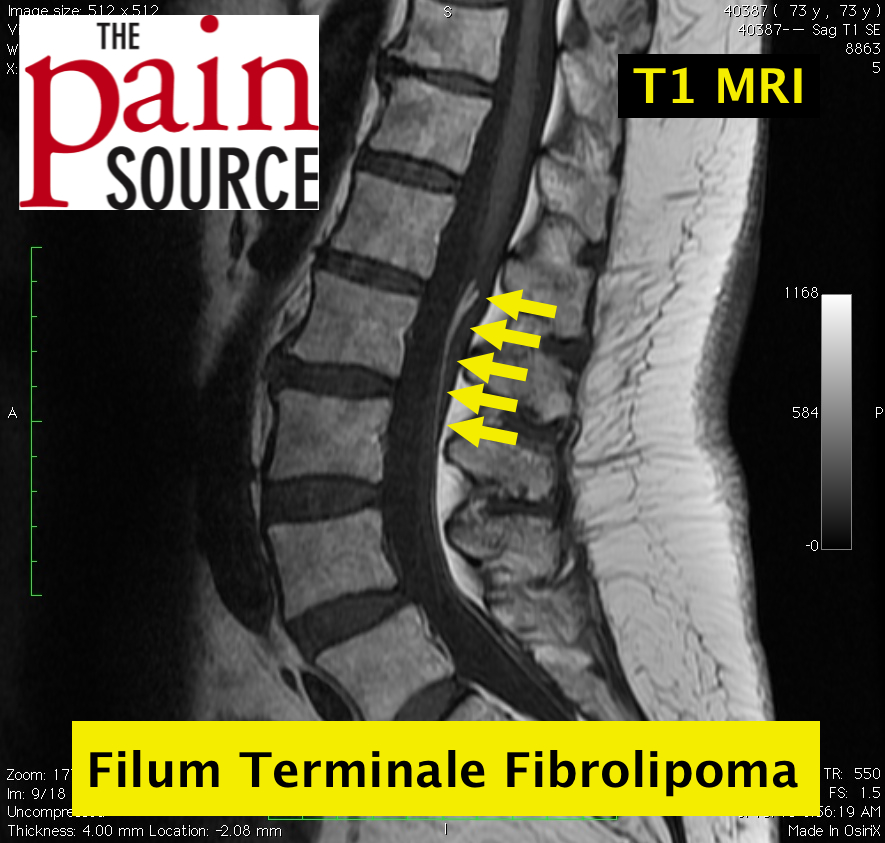
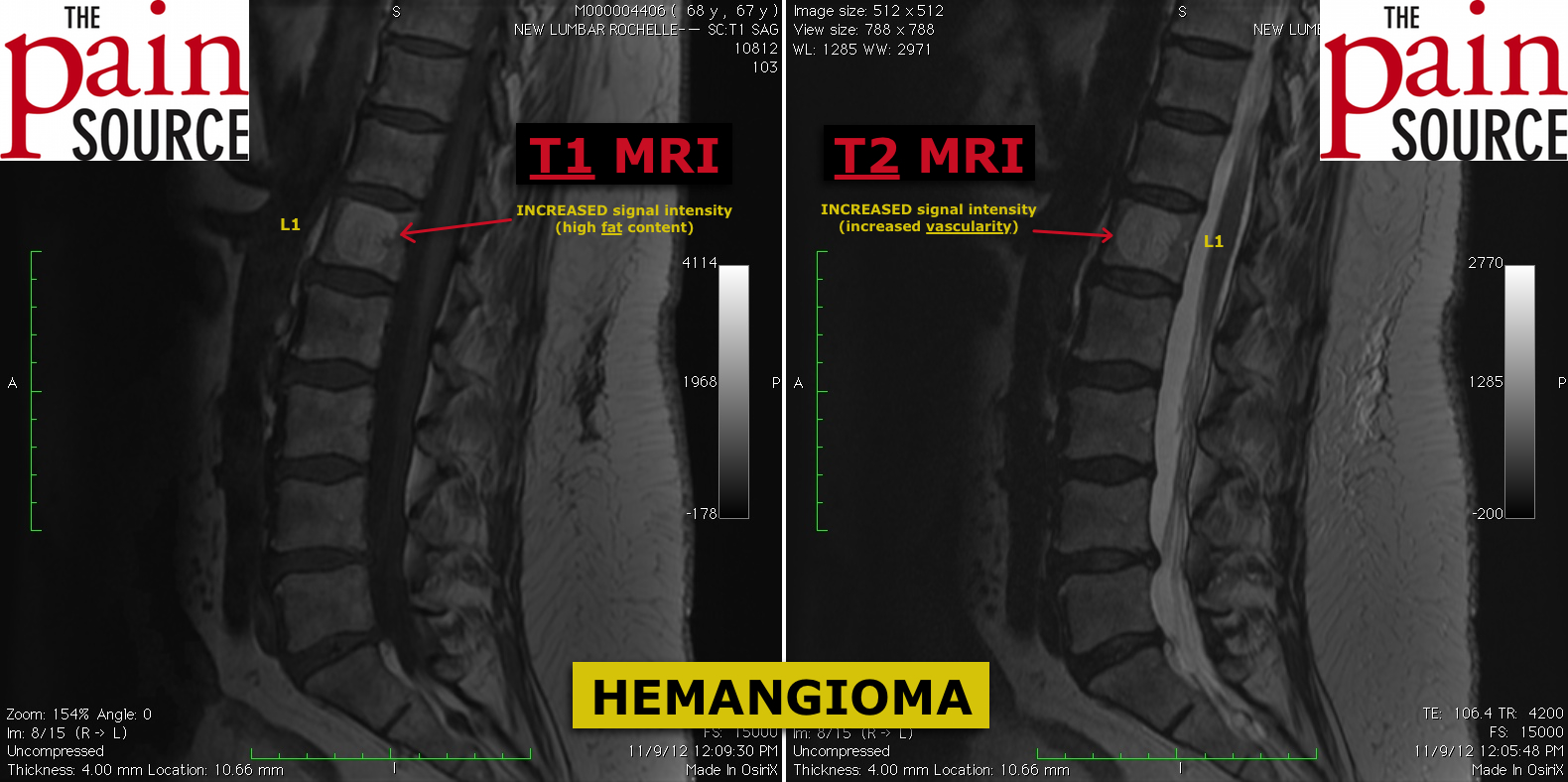
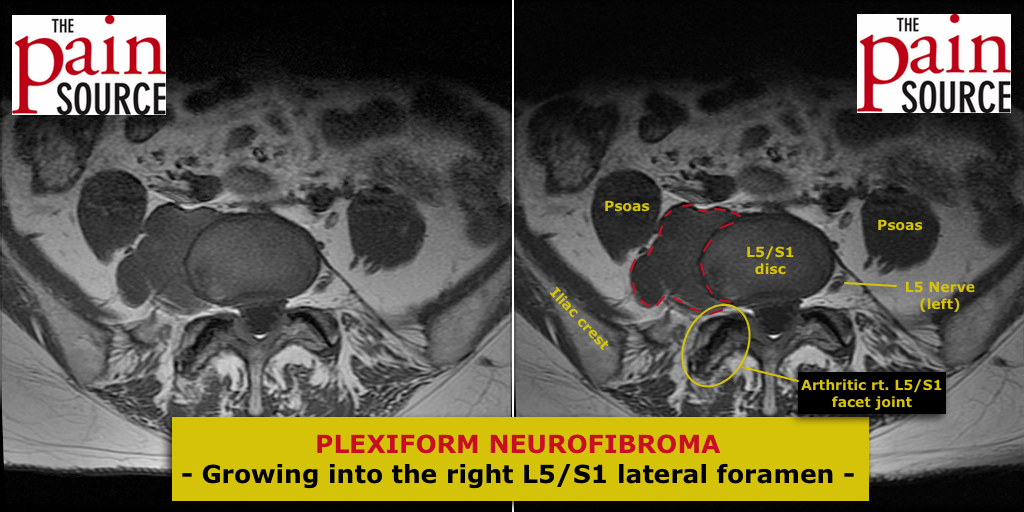
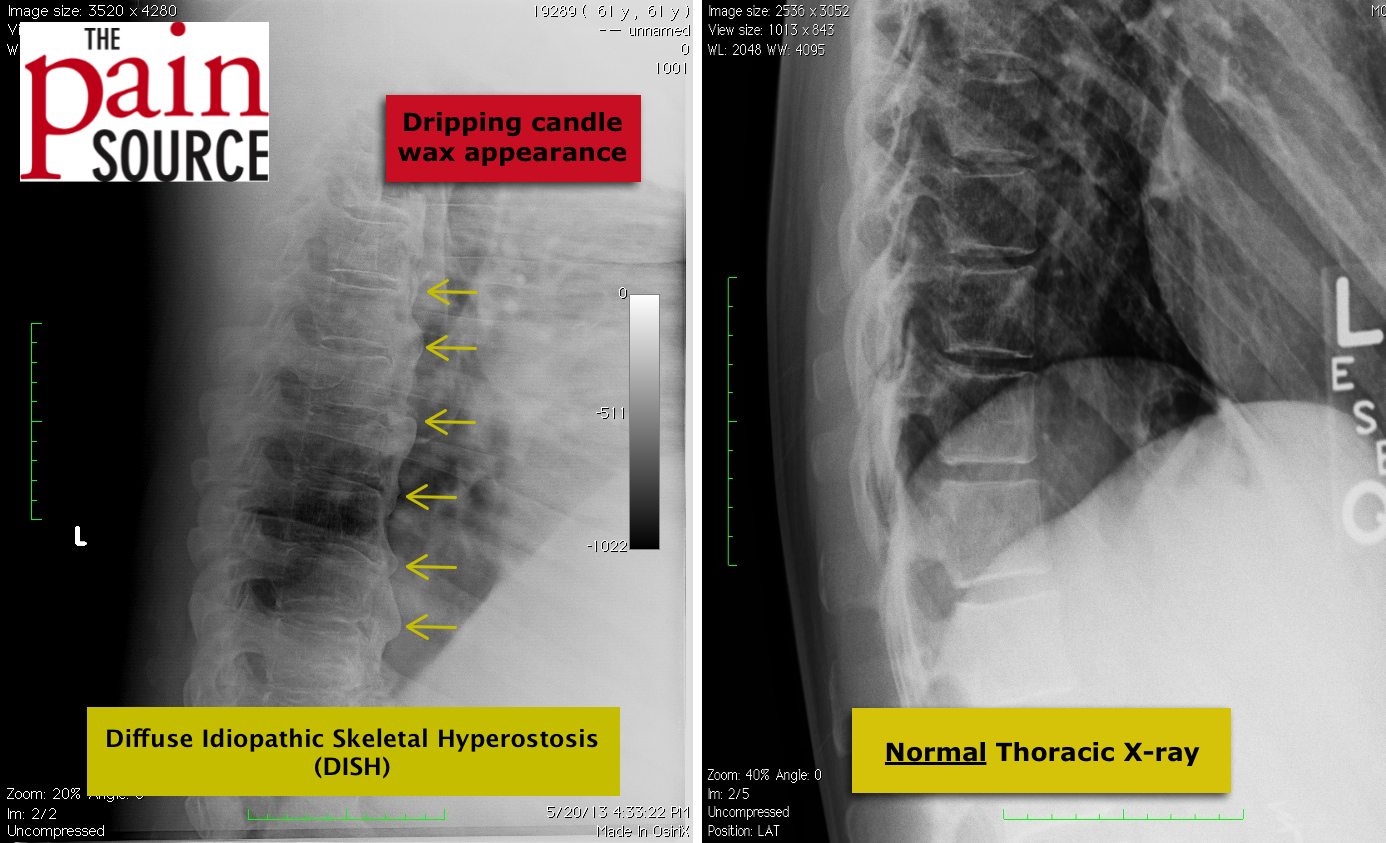
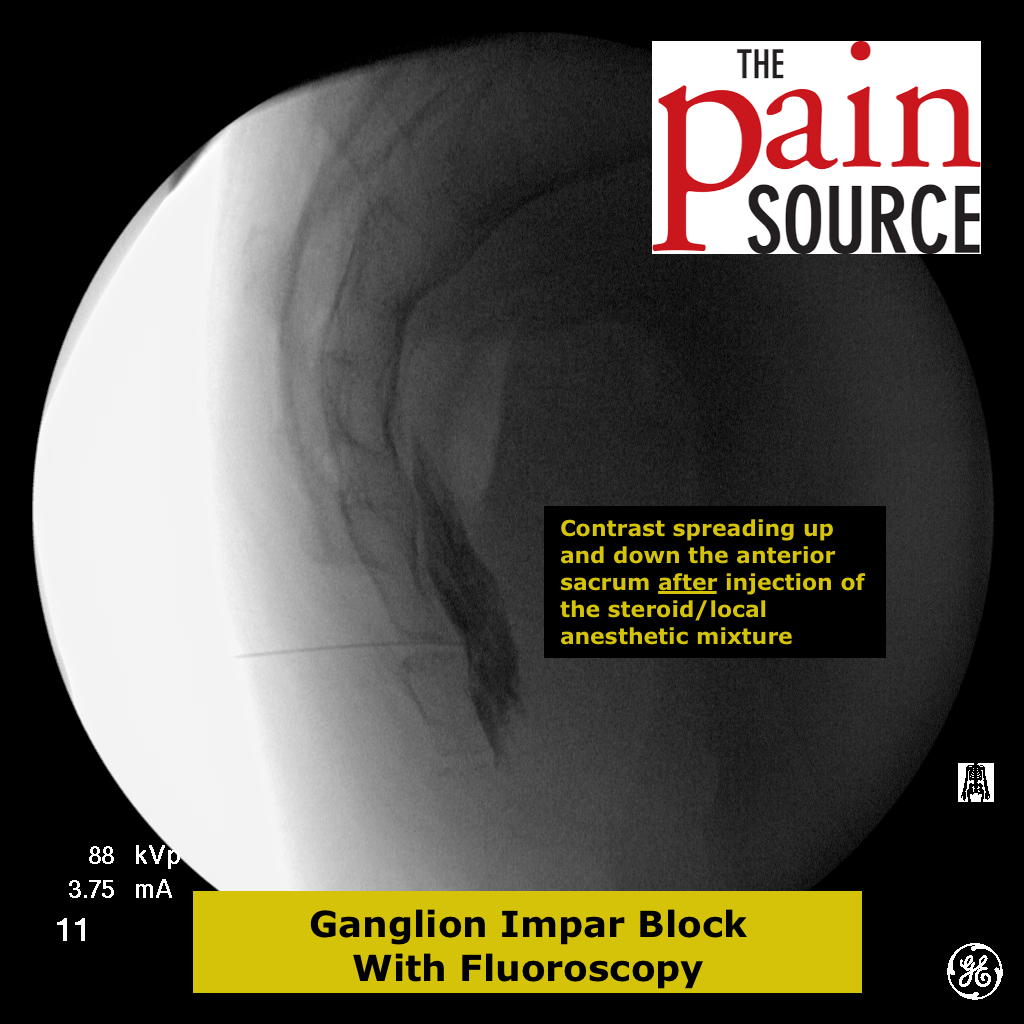
The following information is NOT meant to be used to treat yourself or patients
- Generic name= Pregabalin
- Trade name = Lyrica
- Class = antiepileptic
- MOA
- binds to voltage-gated calcium channels and inhibits neurotransmitter release
- Advantages
- Efficacious against many neuropathic pain conditions
- Linear pharmacokinetics = easier dosing
- Pain relief quicker than gabapentin
- FDA-approved for the treatment of diabetic peripheral neuropathy (2004), postherpetic neuralgia (2004), fibromyalgia (2007), and neuropathic pain associated with spinal cord injury (2012)
- Dosing
- Starting dose: 150 mg/day (divided BID or TID)
- Titrating:
- Increase to 300 mg/day (divided BID/TID) after 3-7 days
- Then, increase by an extra 150 mg/day every 3-7 days
- Max dose: 600 mg/day (200mg TID or 300mg BID)
- Adequate trial period: 4 weeks
- Note: Decreased dose in patients with renal disease
- Note: BID dosing is much more acceptable to patients
- Most common adverse effects
- Peripheral edema and weight gain
- Ataxia, dizziness, somnolence, tremors
- Insurance issues
- Many insurances require failure of gabapentin first. It’s important to note that they may push you to prescribe the patient gabapentin for an off-label Lyrica use such as chronic lumbar radiculopathy, but gabapentin is not FDA approved for that either.
- In my area, BCBS, CareMark, and Express Scripts give us the least trouble with prescribing Lyrica
- Patent expiration on December 30, 2018: http://www.drugs.com/availability/generic-lyrica.html